In the 21st century, with the rise of cell therapy technology, the basic technology of cell line cryopreservation and thawing recovery has been increasingly valued. Cell cryopreservation is a common and very important task in cell culture, just like farmers planting seeds in the field. We need to preserve the cells in their best condition to ensure a continuous supply of seeds for cultivation in the future.
Cell cryopreservation refers to the use of cryopreservation technology to preserve cells at low temperatures, leaving them in a state of temporary detachment from growth, thereby preserving their biological validity for subsequent experiments or clinical use, and facilitating transportation.
In this series of articles, we will discuss the development history of cell cryopreservation technology, traditional cryopreservation methods, and their safety, and the advantages of vitrification cryopreservation technology, fully demonstrating the excellent performance of GMP grade vitrified cell cryopreservation solution developed by Youkang, as well as its safety in clinical applications and drug declaration.
Development history of cell cryopreservation technology
1. In 1776, Spallanzani first published a report on the impact of "cold" treatment on the life activities of "cells".
2. In the mid to late 19th century, many early workers (Prevost, 1840; de Quatrefades, 1853; Mantegazza, 1866; Scheuk, 1870) repeatedly studied the effect of low-temperature treatment on sperm activity, reaching conclusions similar to Spallanzani's. Cold cannot kill sperm.
3. Around 1900, scientists basically confirmed the fact that biological components can be stored at sub-zero temperatures.
4. In the 1950s, multiple scholars such as Luyet discovered the damaging effect of electrolyte concentration on storage cells. Their basic conclusion is that an increase in electrolyte concentration is the main cause of storage cell damage.
5. In the 1960s, Rowe, a blood center in New York, USA, achieved low-temperature preservation of red blood cells. In 1980, he examined red blood cells that had been stored at liquid nitrogen temperature for 12 years and found no biochemical or functional variations, thereby proving in practice that biomaterials can survive for a long time at low temperatures.
6. In the 1970s, Mazur et al. first analyzed the low-temperature preservation experimental data of Chinese hamster tissue cultured cells and proposed a two-factor hypothesis about freezing damage, namely the ice crystal damage and solution damage hypothesis.
7. Since the 1980s, science and engineering have entered the field of low-temperature preservation research, with the continuous updating and application of low-temperature engineering theory and practical design principles, and the rapid development of low-temperature biology research.
8. In 1985, Rail and Fahy successfully vitrified mouse embryos using a certain vitrification solution, marking the first breakthrough in the practical application of this technology.
Since the 20th century, human scientific research has entered the cellular level stage, and low-temperature preservation treatment has been carried out on organisms and biomaterials used as food raw materials. By the end of the 20th century, with the continuous progress of scientific methods and the continuous improvement of freezing methods, low-temperature preservation technology had been widely applied in clinical practice.
Cryopreservation Protectants - Preventing Cell Frostbite
Frozen cells cannot be "let go" at once. Because the water will freeze when it is below zero, the cells will be suspended in the solution. As the temperature decreases, the water outside the cells will freeze first, and the ice crystals formed will cause the destruction of cell membranes and organelles and cause cell death; The electrolyte concentration in an unfrozen solution increases. If cells are exposed to such a high solute solution for too long, lipid molecules on the cell membrane will be damaged, leading to cell leakage. Therefore, a large amount of water will enter the cell during rewarming, causing cell death.
Therefore, it is necessary to add cryoprotectants to the solution during cell cryopreservation to protect the cells from damage. The cryoprotectant is easy to combine with the water molecules in the solution, thus reducing the freezing point, reducing the formation of ice crystals, and reducing its osmotic pressure molar concentration by changing the concentration of electrolyte in the unfrozen solution, so as to protect cells from solute damage.
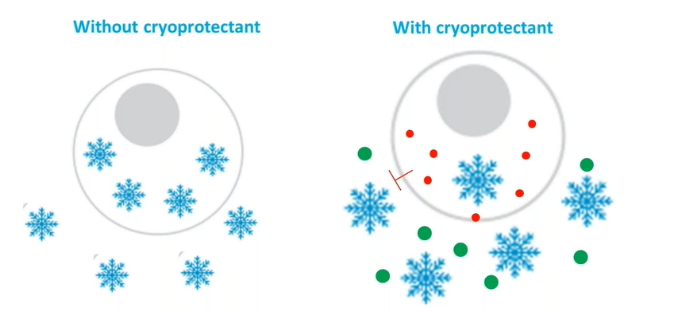
In 1949, Polge et al. discovered that glycerol has a protective effect on cells stored at low temperatures. In 1950, Smith AU successfully used glycerol to freeze red blood cells for the first time and discovered that cryoprotectants were a key factor affecting the cryopreservation effect. The period from 1949 to 1960 can be referred to as the "glycerol period" of cryopreservation, during which glycerol is generally used as a protective agent for the cryopreservation of biological materials. In 1959, Lovelock et al. discovered a new chemical protectant, which is known as dimethyl sulfoxide (DMSO) and has been used to this day.
The commonly used formula for cell cryopreservation protection in the laboratory is 10% dimethyl sulfoxide (DMSO)+90% fetal bovine serum (FBS), which has good universality and cell protection effect, and has been widely recognized. However, there is currently no DMSO product of medicinal injection grade. In addition, this formula contains FBS from animal sources, which poses a safety hazard that cannot be eliminated. Whether it can be directly applied in clinical practice is still a question worth exploring.
In this article, we discussed the development history of cell cryopreservation technology and the necessity of the emergence of cryoprotectants. We also briefly mentioned the commonly used formulas for cell cryoprotectants. In the next article, we will delve into the safety issues of commonly used cell cryopreservation solutions in clinical practice.
Ji Guangchao, Wang Xiaoming, Wang Yuqi, Song Hangfei. Experimental exploration of clinical cell cryopreservation protective solution [J]. Chinese Medical Biotechnology, 2021,16 (02): 158-160
Qiu Jiaying, Jia Xiaoqing, Huang Gang, Tan Shuhua. Research progress in cell and tissue cryopreservation methods and applications [J]. Chinese Journal of Biological Products, 2017,30 (05): 546-550. DOI: 10.13200/j.cnki. cjb.001743
Application direction, development history, and freezing technology of cell cryopreservation and resuscitation.

 Yocon Market
Yocon Market 2023-06-02
2023-06-02 Company Events
Company Events


