“Accurate, fast, convenient and secure”
This is a prioritization of our requirements for the workstation.
The workstation has to be put into operation.
One of the biggest concerns that customers often have about such product is that -
Whether the equipment will produce a false positive result (cross-contamination of different samples)?
To make customers more assured, we have tested the FE4800 automatic nucleic acid extraction workstation, using real virus samples (purchased from national institutions through regular channels, without infectivity), and have made the first success!
I. Background
What is the possible cause for cross-contamination of samples?
--Too short distance between sampling tubes and between different deep-well plates, and inevitable aerosol produced during liquid transfer by equipment. Cross-contamination of samples may occur if aerosols from one sample fall into another. Our equipment processes 8 samples simultaneously, and it is normal for customers to have doubts.
Why should we prevent such cross-contamination?
--Not all cross-contaminations have serious consequences, but cross-contamination in the molecular biology does produce extremely serious consequences because the back-end testing principle is multiple replication of the initial sample. Based on the current CT value of 35 as the cut-off value for a positive or negative diagnosis during the clinical test, the initial sample should be replicated 235 times, that is, 64.0 billion times. It is amazing that one nucleic acid can be amplified to 64.0 billion nucleic acids. Therefore, whenever a sample is contaminated by another one, result errors are inevitable.
2.Objective
To systematically evaluate the performance of YOCON automatic sample processing and nucleic acid extraction workstation and to verify that there is no aerosol contamination during normal extraction by the equipment.
3.Design
1、 A large number of strongly positive samples and negative samples were arranged in a staggered manner for replicate tests of 3 batches to test whether the extraction equipment would lead to cross-contamination under extreme conditions.
2、 A total of 48 samples were extracted for one batch, including 27 virus-containing samples and 21 virus-free samples.
3、 The virus concentration of the positive samples was 109 copies/mL (refer to the upper limit of actual concentration of positive clinical samples).
4、 Virus-containing samples and blank samples were arranged in a staggered manner, with positive samples around negative sample (negative-6 sample as shown below) and negative samples around positive sample (2-1 sample as shown below), as detailed below:
Arrangement of negative and positive samples.
Negative | Negative | Negative | Positive | Negative | Positive | Negative | Positive |
Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive |
Negative | Negative | Negative | Positive | Positive | Negative | Positive | Negative |
Positive | Positive | Positive | Positive | Positive | Positive | Positive | Negative |
Positive | Negative | Positive | Positive | Positive | Negative | Positive | Positive |
Positive | Positive | Positive | Positive | Negative | Negative | Negative | Negative |
4.Procedures
1、 The virus mixture was prepared.
2、 The virus samples were added. The virus mixture was added to the sampling tube in a 1:9 volume ratio. A total of 27 virus-containing sampling tubes were set, marked with serial numbers, and recorded. One swab was broken and put into each sampling tube. The tubes were shaken well and mixed well.
3、 Preparation of the reagents required for the instrument. A total of three reagents were required, that is, 15.36 mL of a mixture of isopropanol and magnetic beads (containing 14.4 mL of isopropanol and 960 μL of magnetic beads), 38.4 mL of purification solution and 4.8 mL of cleaning solution.
4、 27 tubes were inserted into the pre-set wells from left to right and top to bottom, and the negative control tube without virus sample were inserted into the remaining 21 wells to start the program for extraction.
5、 The reverse transcription fluorescence quantitative PCR assay was carried out for 21 negative samples, and one well containing viral nucleic acid was set as positive control.
6、 The replicate tests were carried out for 3 batches.
5.Results
1. The expansion curves of each group for batch 1 are as follows:
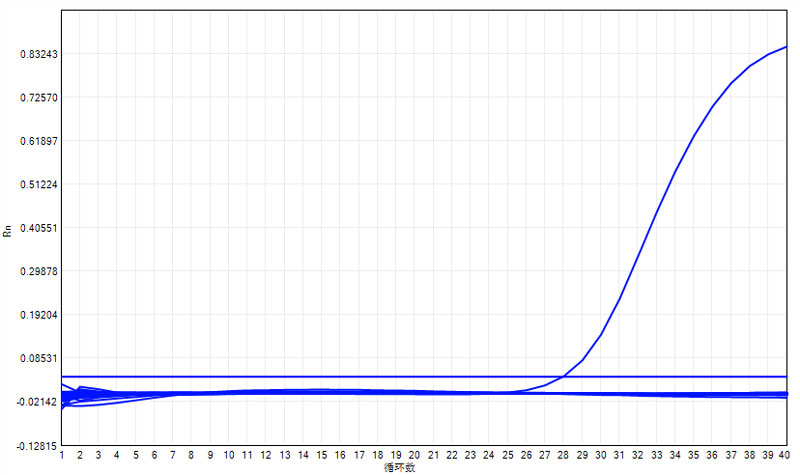
Note: Only the positive control group had an expansion curve, while the negative control and others showed no expansion.
Statistics:
Group | Ct value | Group | Ct value | Group | Ct value |
Negative-1 | NoCt | Negative-8 | NoCt | Negative-15 | NoCt |
Negative-2 | NoCt | Negative-9 | NoCt | Negative-16 | NoCt |
Negative-3 | NoCt | Negative-10 | NoCt | Negative-17 | NoCt |
Negative-4 | NoCt | Negative-11 | NoCt | Negative-18 | NoCt |
Negative-5 | NoCt | Negative-12 | NoCt | Negative-19 | NoCt |
Negative-6 | NoCt | Negative-13 | NoCt | Negative-20 | NoCt |
Negative-7 | NoCt | Negative-14 | NoCt | Negative-21 | NoCt |
Positive control | 27.95 | Negative control | NoCt |
2. The expansion curves of each group for batch 2 are as follows:
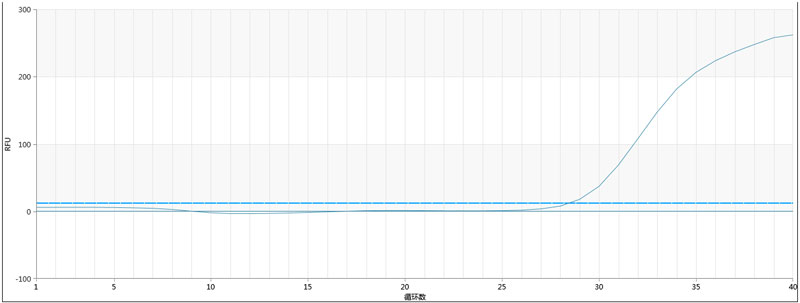
Note: Only the positive control group had an expansion curve, while the negative control and others showed no expansion.
Statistics:
Group | Ct value | Group | Ct value | Group | Ct value |
Negative-1 | NoCt | Negative-8 | NoCt | Negative-15 | NoCt |
Negative-2 | NoCt | Negative-9 | NoCt | Negative-16 | NoCt |
Negative-3 | NoCt | Negative-10 | NoCt | Negative-17 | NoCt |
Negative-4 | NoCt | Negative-11 | NoCt | Negative-18 | NoCt |
Negative-5 | NoCt | Negative-12 | NoCt | Negative-19 | NoCt |
Negative-6 | NoCt | Negative-13 | NoCt | Negative-20 | NoCt |
Negative-7 | NoCt | Negative-14 | NoCt | Negative-21 | NoCt |
Positive control | 28.55 | Negative control | NoCt |
3. The expansion curves of each group for batch 3 are as follows:
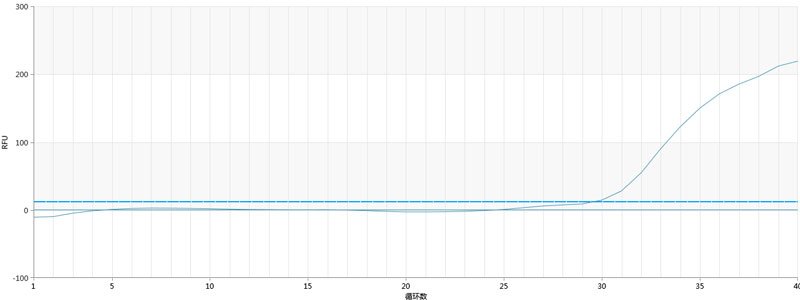
Note: Only the positive control group had an expansion curve, while the negative control and others showed no expansion.
Statistics:
Group | Ct value | Group | Ct value | Group | Ct value |
Negative-1 | NoCt | Negative-8 | NoCt | Negative-15 | NoCt |
Negative-2 | NoCt | Negative-9 | NoCt | Negative-16 | NoCt |
Negative-3 | NoCt | Negative-10 | NoCt | Negative-17 | NoCt |
Negative-4 | NoCt | Negative-11 | NoCt | Negative-18 | NoCt |
Negative-5 | NoCt | Negative-12 | NoCt | Negative-19 | NoCt |
Negative-6 | NoCt | Negative-13 | NoCt | Negative-20 | NoCt |
Negative-7 | NoCt | Negative-14 | NoCt | Negative-21 | NoCt |
Positive control | 29.72 | Negative control | NoCt |
6.Conclusions
During the extraction tests for the same batch, negative samples and positive samples were arranged in a staggered manner, and contamination was not found in negative sample wells, but found in positive control well, indicating that this equipment can effectively prevent inter-sample cross-contamination.
Words in the end:
We choose to be one of the few automatic nucleic acid workstation suppliers to offer measured data of samples using our automatic nucleic acid workstation. We will continue to test multiple batches of samples in the future to ensure that the equipment meets the customer needs. Let’s look forward to it!

 Yocon Market
Yocon Market 2021-10-20
2021-10-20 Company Events
Company Events


